Oral glucose tolerance test (OGTT)
Objectives
Oral glucose tolerance test is commonly used to monitor how blood glucose homeostasis is maintained following glucose overload. This test is complementary to glycemia monitoring for diabetes care and could be necessary to detect more subtle changes during the development of insulin resistance.
Summarized methodology
• Briefly, after overnight fasting, a solution of glucose 1 g/kg is administered by gavage. Blood samples are taken at 0, 10, 20, 30, 60 and 90 minutes after the gavage.
• Plasma glucose and insulin are determined for each time point.
Endpoints
• Evaluation of blood glucose / insulin metabolism following glucose overload (figure 1).
• Determination of the insulin sensitivity index (ISI) calculated from glycemia and insulinemia evolution profile (figure 1).
• Efficacy of a treatment targeting blood glucose metabolism.
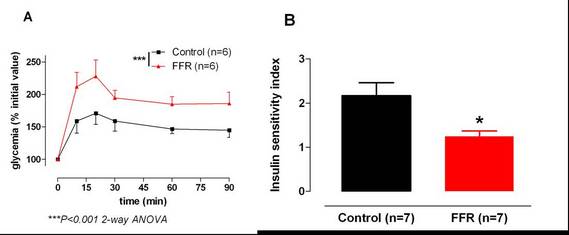 Figure 1: Glycemia monitoring during OGTT (A) and resulting insulin sensitivity index (B) in control rats and in an experimental model of insulin resistance: the fructose-fed rat (FFR) (Pelvipharm internal data and Oudot et. al 2009).
Figure 1: Glycemia monitoring during OGTT (A) and resulting insulin sensitivity index (B) in control rats and in an experimental model of insulin resistance: the fructose-fed rat (FFR) (Pelvipharm internal data and Oudot et. al 2009). NB: Pelvipharm will gladly study the feasibility of performing OGTT in other experimental models to meet its client’s needs.
Oudot, A. et al. Physiol Res (2009) : 58(4):499-509
Behr-Roussel, D. et al. Eur Urol (2008) : 53(6):1272-1281
Behr-Roussel, D. et al. Am J Hypertens (2008) : 21(11):1258-1263

Links to applicable Targeted disorders / Pathophysiological models




















 Download this page in PDF
Download this page in PDF